On your busy overnight trauma shift at Elmhurst, you are the provider during a precipitous vaginal delivery in the trauma bay at 2am. The infant is delivered without complication, and your Pediatric colleagues give you APGAR’s of 8 and 9. As you call up to L&D, you are told that the staff; attendings and residents included, are in a stat C -section and will come down to evaluate the patient afterwards. The patient begins to have brisk vaginal bleeding after the delivery, so you begin Pitocin intravenously and attempt to deliver the placenta. After a successful delivery of an intact placenta, the patient continues to have brisk heavy vaginal bleeding. She has lost at least 1300cc’s of blood. Her BP has now dropped to 88/55. In addition to managing the ABC’s, what is your next move? Enter The WOMAN Trial.
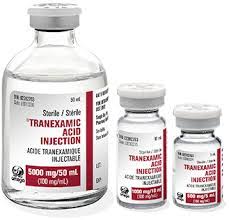
WOMAN Trial: [World Maternal Antifibrinolytic Trial] Tranexamic acid for the treatment of postpartum hemorrhage: an international randomized, double blind placebo controlled trial
Background: Postpartum hemorrhage (PPH) is the leading causes of maternal mortality worldwide, and is highly prevalent in resource poor countries.
Question: Will the administration of TXA, when compared to a placebo reduce mortality in women diagnosed with postpartum hemorrhage (>500ml within 24 hours of giving birth) ?
Design, setting, population: Double blinded randomized control trial, 193 hospitals in 23 countries between 2010 – 2016, women aged 16 and over, diagnosed with PPH after vaginal birth or C-section, 20,060 women enrolled with even distribution of patient characteristics between intervention and placebo groups.
Intervention: 1g TXA IV
Control: Placebo – given IV in an identical bottle
Outcomes:
Primary outcome: All cause mortality within 42 days: 5.5% in placebo group vs 5.3% in TXA group [95% CI 0·87-1·09(p= 0.65)]
Secondary outcomes: Death due to bleeding = 1.9% in placebo group vs 1.5% in TXA group. [95% CI 0·65–1·00(p= 0.045)] ARR 0.4%, NNT 267. TXA given within 3 hours of giving birth reduced mortality; 1.7% in placebo group vs 1.2% in TXA group. [RR 0·69, 95% CI 0·52–0·91(P= 0.008)]
The Take away: TXA is a relatively inexpensive drug that is readily available even in resource poor areas. Although there was no significant reduction in all cause mortality, administration of TXA did reduce death due to bleeding and can be added to your armamentarium of tools to manage life threatening PPH in the ER.
References: