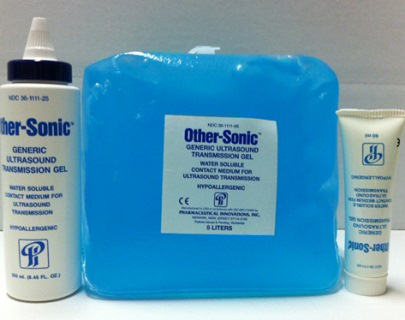
On April 18 the FDA released an alert regarding Other-Sonic Generic Ultrasound Transmission Gel, manufactured by Pharmaceutical Innovations Inc. The ultrasound gel was found to be contaminated with Pseudomonas aeruginosa and Klebsiella oxytoca.
According to the FDA Press Announcement,
U.S. Marshals, acting at the request of the Food and Drug Administration, have seized Other-Sonic Generic Ultrasound Transmission Gel located at Pharmaceutical Innovations Inc. in Newark, N.J., after an FDA analysis found that product samples contained dangerous bacteria. The seizure included all lots of the gel product manufactured between June 2011 and December 2011….The FDA received a report involving 16 surgical patients infected with Pseudomonas aeruginosa. The patients had transesophageal ultrasound procedures, while undergoing heart valve replacement, using Other-Sonic Generic Ultrasound Transmission Gel.
Yes, that first line said “U.S. Marshals.” The FDA does not mess around. So take a minute and check your gel! Maybe a good time to wipe the whole machine down while you are at it.