But what is the pH?
Written by Erena Weathers
Per direct request: “Some people can just look at a VBG and know what’s going on. I want to be able to do that!”
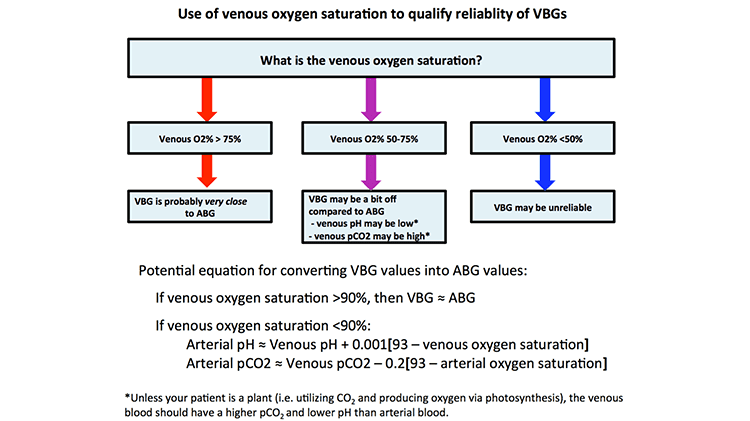
First, big ups to Dr. Hashem Zikry of “Runs Really Fast” Fame (2:32:51 2018 NYC Marathon time) for his TR pearl of how you can reliably use a VBG in place of ABG. See Zeserson et al for more info.
With that let us begin.
pH – normal 7.35 – 7.45
The body’s pH is closely controlled through buffering and excretion of acids.If you note a value outside this range, there is an acid base disturbance. <7.35 acidotic, and >7.45 alkalotic. To determine if respiratory vs metabolic take a look at the next value.
pCO2 – normal 41-54
This is what you should be using to determine respiratory acidosis/alkalosis since the bicarb is a calculated value on the VBG.
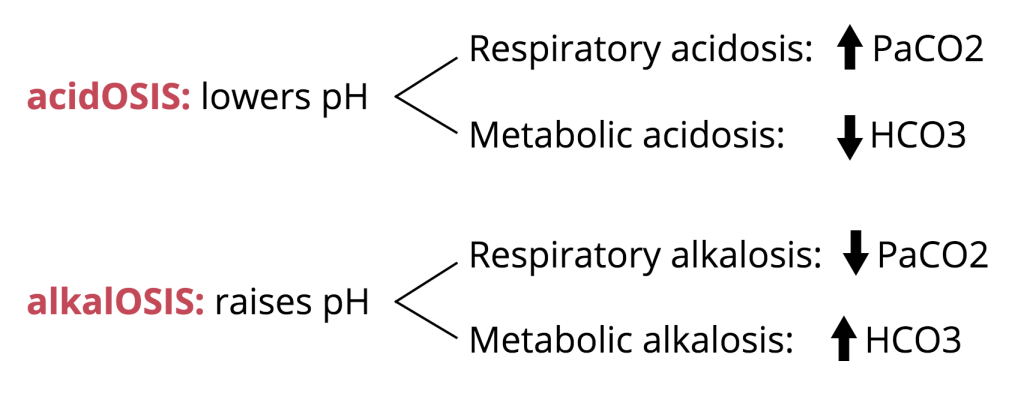
However, the pH can be normal and still have a metabolic derangement. Say if a patient has a pH of 7.35 but pCO2 12 then they have a mixed respiratory alkalosis with another acid/base disturbance. In order to determine that, you need a formal BMP since the bicarb on the VBG is a calculated value.
Another thing you can use the pCO2 is to see if the patient is adequately compensating for a metabolic acidosis. You can either use Winter’s formula (Expected PaCO2 = (1.5 x serum HCO3)+(8±2)) or Expected PaCO2 = last two digits of the pH ± 2. I go with the second one because it’s easier.
pCO2 is also used to track hypercarbia, as in monitoring your COPD patients. When in Resus taking care of a COPD exacerbation you want to trend the pCO2 to insure it is decreasing with your intervention. You generally want to target a pCO2 close to the patient’s baseline value and if you do not know their baseline then pH 7.25-7.35 and/or clinical improvement.
Electrolytes Pouryahya P, Tan L, Lin ZC, Meyer A. Reliability of venous blood gas sodium, potassium and creatinine. N Z Med J. 2018 Dec 14;131(1487):38-43. PMID: 30543610.
K, Na, and Cr are closely correlated in a (non-hemolyzed) VBG and with a formal BMP in those with a normal pH. In acidotic patients, the K has a lower correlation in a VBG than in a BMP. You can use these as quick markers of important electrolyte abnormalities. Be wary, VBG doesn’t take into account hemolysis for K values so use your clinical judgment.
Glucose Self-explanatory
Lactate – normal <2
Can help determine if a patient is acidotic, if lactic acidosis is contributing to the problem.
Hb/Hct
Still need CBC and T+S prior to transfusion in stable patients. Can be helpful in starting the process of blood transfusion, since that can be a pain.
Carboxyhemoglobin – normal <2.9%
The average non-smoker maintains a COHb level under 3% COHb whereas smokers approach 10% COHb. If concern for CO toxicity, >25% absolute indication for hyperbaric, and >15% for pregnant patients.
Methemoglobin – normal <1.5%
Methemoglobinemia is suspected in a child or adult with unexplained cyanosis or hypoxia that does not resolve with supplemental oxygen and/or suspicious history. This value just helps you when you report it to poison control and to trend.
Base Excess – normal -2 to +2
The BE is the amount of strong acid which would need to be added or subtracted in order to return to a normal pH. A value outside of the normal range suggests a metabolic cause for the acidosis or alkalosis. A value greater +2, would indicate a metabolic alkalosis. A value less than -2, would indicate a metabolic acidosis.
That was a whirlwind and a lot. Hopefully, VBGs became slightly more digestible.
References
Pouryahya P, Tan L, Lin ZC, Meyer A. “Reliability of venous blood gas sodium, potassium and creatinine.” N Z Med J. 2018 Dec 14;131(1487):38-43. PMID: 30543610.
Farkas J. “PulmCrit – How to convert a VBG into an ABG.” PulmCrit (EMCrit). 16 Jan 2017. https://emcrit.org/pulmcrit/vbg-abg/
Zeserson E, Goodgame B, Hess JD, et al. “Correlation of Venous Blood Gas and Pulse Oximetry With Arterial Blood Gas in the Undifferentiated Critically Ill Patient.” J Intensive Care Med. 2018;33(3):176-181. doi:10.1177/0885066616652597
