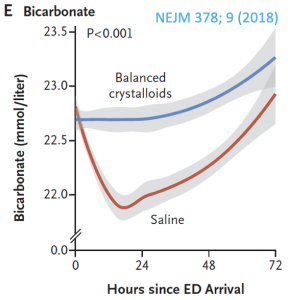
Previous post reviewed the safety of balanced crystalloids in hyper K. But what was up with serum bicarbonate decreasing with saline administration?
 This post introduces a new way of looking at the anion gap to possibly answer this phenomenon. This is the way we normally think of the anion gap (composed of anions like phosphate, albumin, Ig, etc) with respect to the other major extracellular ions, but what if bicarbonate were a dependent factor?
This post introduces a new way of looking at the anion gap to possibly answer this phenomenon. This is the way we normally think of the anion gap (composed of anions like phosphate, albumin, Ig, etc) with respect to the other major extracellular ions, but what if bicarbonate were a dependent factor?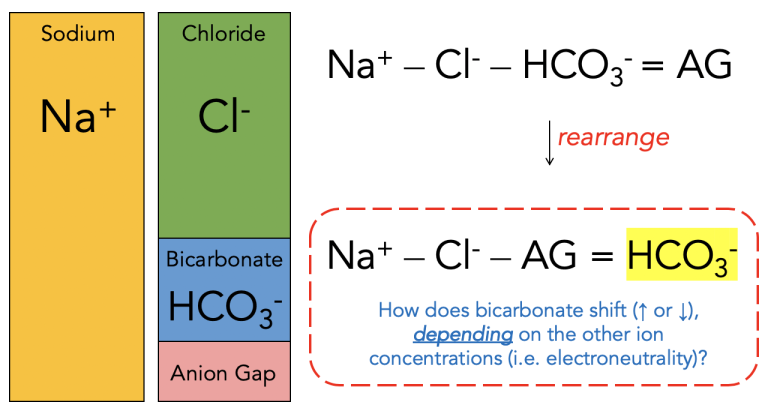
Now consider if saline is given (c.f. table of fluid composition), which has higher Cl- content; or if a patient has vomited a lot, which decreases gastric H+ and Cl-:
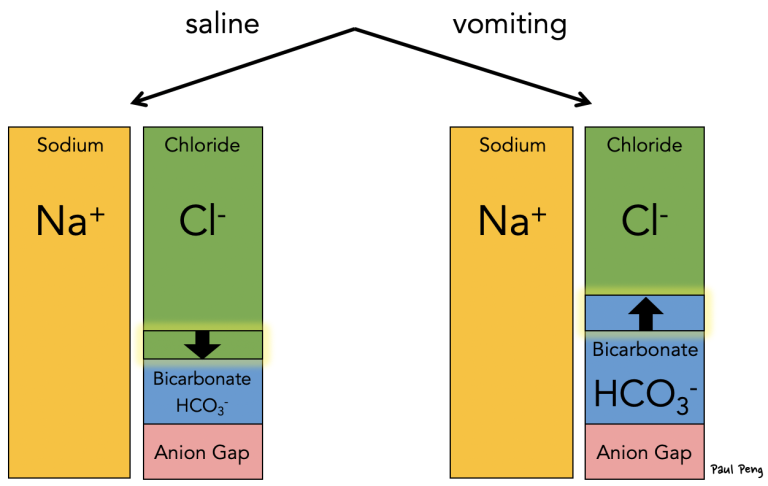 In the case of saline, the resultant decrease in bicarbonate (squeezed down by the increased chloride) leads to a non-anion gap metabolic acidosis… and that’s why saline causes a hyperchloremic acidosis.
In the case of saline, the resultant decrease in bicarbonate (squeezed down by the increased chloride) leads to a non-anion gap metabolic acidosis… and that’s why saline causes a hyperchloremic acidosis.
Considering bicarbonate as a dependent variable is one of the concepts behind the Stewart approach to acid-base physiology, as opposed to the more familiar Henderson-Hasselbalch. The Stewart approach also places emphasis on the strong ion difference (SID). Here is my approach to thinking about acid-base:
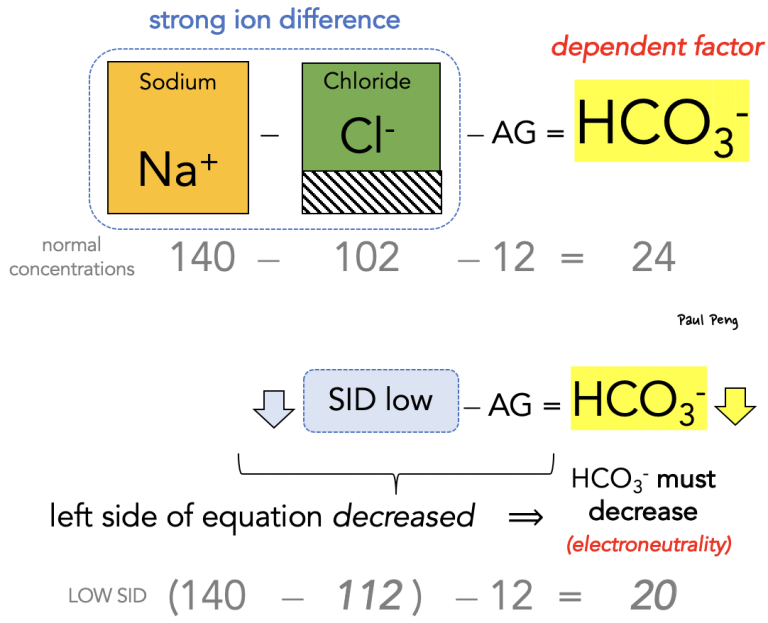 In other words, any fluid that causes the strong ion difference to decrease (e.g. saline) will make the left-hand side of the “new” anion gap equation smaller, which has to be balanced by a similar decrease in bicarbonate.
In other words, any fluid that causes the strong ion difference to decrease (e.g. saline) will make the left-hand side of the “new” anion gap equation smaller, which has to be balanced by a similar decrease in bicarbonate.
On a related note, what is contraction alkalosis?
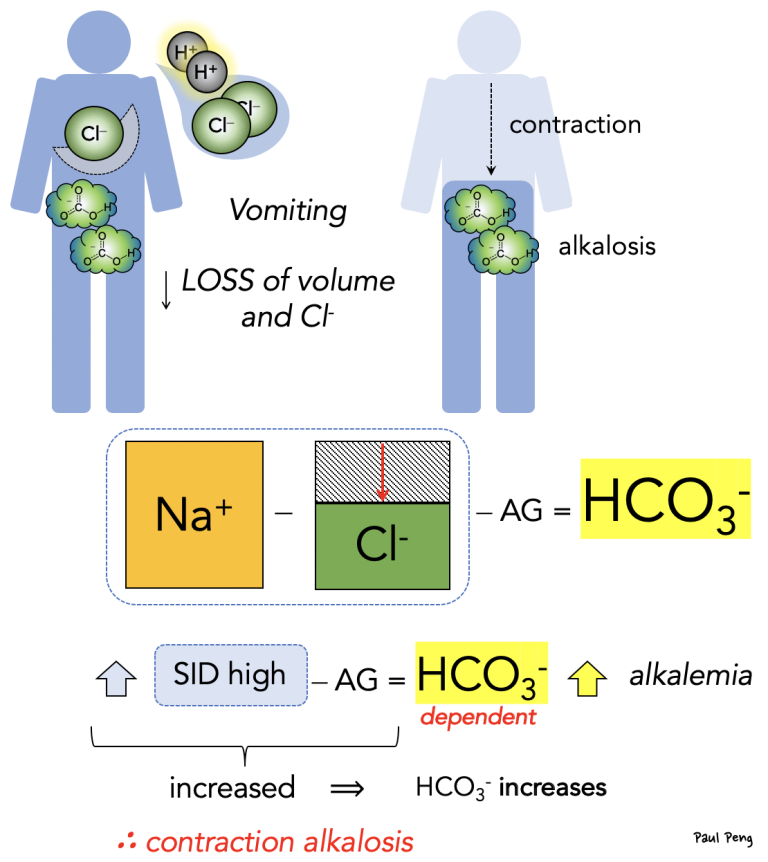 Not only is there less volume for the remaining bicarbonate (hence a higher concentration), but the loss of chloride leads to a higher SID, which is balanced by an increase in bicarbonate, causing a (contraction) metabolic alkalosis.
Not only is there less volume for the remaining bicarbonate (hence a higher concentration), but the loss of chloride leads to a higher SID, which is balanced by an increase in bicarbonate, causing a (contraction) metabolic alkalosis.
Back to saline and hyperchloremic acidosis…
Hyperchloremia also causes RENAL VASOCONSTRICTION. This is possibly contributory to the higher incidence of major adverse kidney events at 30 days in patients who received normal saline (SALT-ED trial):